Monoclonal Antibodies, Nova Scotia's 'Unspent Ammunition'
Part 2: The COVID-briefings, a leaked document, and an update
I registered for my first COVID-briefing on November 24th.
As I laid out in Part 1 of this series, one of the reasons provided to me for not deploying bamlanivimab during the 3rd wave in Nova Scotia was that the drug had been rendered incapable of neutralizing the variant of the virus that was circulating at the time. Recall, in May 2021, Dr. Lisa Barrett, a prominent Nova Scotia clinician and co-chair of the Therapeutics and Prophylactics Advisory Group said in an interview that the variant that was here in the province was a “late” UK/ ALPHA variant.
But, as previously mentioned, the manufacturer of the treatment, Eli Lilly, said there were no reports of this further mutated ALPHA variant in circulation in Canada at that point. Eli Lilly’s contention was corroborated by Canada’s database on Variants of Concern (VOC) as well as the minutes of the Advisory Group obtained through Freedom of Information (FOI), which not only made no mention of a “late” variant but stated that bamlanivimab maintained its effectiveness at neutralizing the virus that was dominant in the province. Furthermore, even a Health Canada warning about bamlanivimab, which stated there was a “potential risk of treatment failure” with certain VOCs, stated clearly that it was “expected to retain neutralizing activity against the UK origin B.1.1.7 variant.”
Despite all this, bamlanivimab was provided to only one individual during wave 3.
But as ALPHA gave way to DELTA, Health Canada was authorizing new monoclonals for emergency use: in June, casirivimab and imdevimab (Hoffmann-La Roche), got the go-ahead, and at the end of July so did sotrovimab (GlaxoSmithKlein). Both products were found to be effective against the DELTA variant and, once again, were found to result in significantly lower rates of hospitalization.
But despite this, the Advisory Group still did not recommend them in “routine care.” Like bamlanivimab, they were only to be used in “pragmatic research,” that is in clinical trials such as the one Dr. Barrett was heading up. However, from what I could gather, the treatment was never used in that context either.[i]
By the time November rolled around, I was surprised there was still no uptake on this story by other reporters in this province (and for what it’s worth, there still isn’t). Even my own attempt at getting my follow-up piece published in The Halifax Examiner ran into a brick wall. Instead, I posted it on my Web site, which has meagre reach and about as much traffic as a rural Nova Scotia road at 3am on a weeknight.
But then I realized that one way to get the information out to a wider audience was to register for a COVID-briefing. A lot of people watch and listen to the live-stream. So, on November 24th I asked Dr. Strang the following question:
During both the 3rd wave and now the 4th, there have been Health Canada-authorized monoclonal antibody treatments, meant to be provided early, for people who come down with COVID and are at high risk of serious progression and hospitalization. During the 3rd wave the province had 150 doses of bamlavinimab, which was effective against the ALPHA or UK variant, which was mainly circulating here at that time – but only one dose was used. Health Canada recently sent Nova Scotia new monoclonals, effective against the DELTA variant. Saskatchewan and Alberta have made the treatment available to those at high risk – in Alberta it’s being administered by paramedics in people’s homes. There’s also a pilot project in Hamilton, Ontario. My question is, why aren’t these treatments being offered routinely to Nova Scotians to help keep them out of hospital, and potentially save lives?
Dr. Strang replied that the question was best directed to the Therapeutics and Prophylactics Advisory Group, which provides recommendations on early treatment options. Dr. Strang likely didn’t know I had been steeped in the story for months; had filed FOI requests and was in possession of nearly 1,000 pages, most of them highly redacted. Even so, I wasn’t any closer to understanding why Nova Scotians were not given access to these early treatments.
Around the same time I also sent an opinion piece to Saltwire, which published the piece a few days later, and aptly titled it, “Unspent Ammunition.”
A couple of weeks passed.
On December 7, I registered again for a COVID briefing and asked the following:
Dr. Strang, a couple weeks ago I asked you why federally authorized monoclonal antibodies, effective against the DELTA variant, were not being offered to high-risk Nova Scotians as an early treatment for COVID, and you deferred to Dr. Lisa Barrett and the Therapeutics and Prophylactics Advisory Group. I’ve reached out to them over the last few months, but I’m still not any closer to having answers. I also recently contacted the NS Health Authority to find out if these treatments were deployed at the Long-Term Care Facility in Pugwash, the site of a recent outbreak linked to the faith gatherings, and received no response. Dr. Strang, you also sit as a non-voting member on the Advisory Group, what can be done to ensure those Nova Scotians at the highest risk of hospitalization get access to this potentially life-saving treatment?
Dr. Strang replied:
“What I can tell you is we are using the monoclonal antibodies for select patients, for those it’s deemed appropriate. Again I defer to the clinicians who are responsible for treating patients and I would encourage you to continue to ask the Nova Scotia Health Authority.”
So I followed up again with Carla Adams (Nova Scotia Health Authority) to find out more about how it was being used, and she sent me the following reply from Barrett:
“To date casirivimab and imdevimab have been used four times for immunocompromised patients.”
Barrett also had some new information to pass along:
“Public Health is developing a screening tool to help identify potential candidates for monoclonal antibody therapy in the community, where eligible Nova Scotians would be referred to a group of COVID-19 clinicians for assessment and treatment.”
Along with this development, I learned that the Advisory Group had also changed their recommendation on the use of monoclonals to “routine care.”
I asked Adams if the government would be informing the public about the availability of these early treatments and she replied, “I would not expect that we would announce it as it hasn’t been our practice.”
But other provinces, namely Saskatchewan and Alberta have online tools the public can use to determine their eligibility for the treatments. The Ontario Science Table had publicly announced its recommendations for the use of monoclonals in adults. The Web site for the Hamilton-based monoclonal clinic started by Dr. Zain Chagla provides a tool that can be used by members of the public.
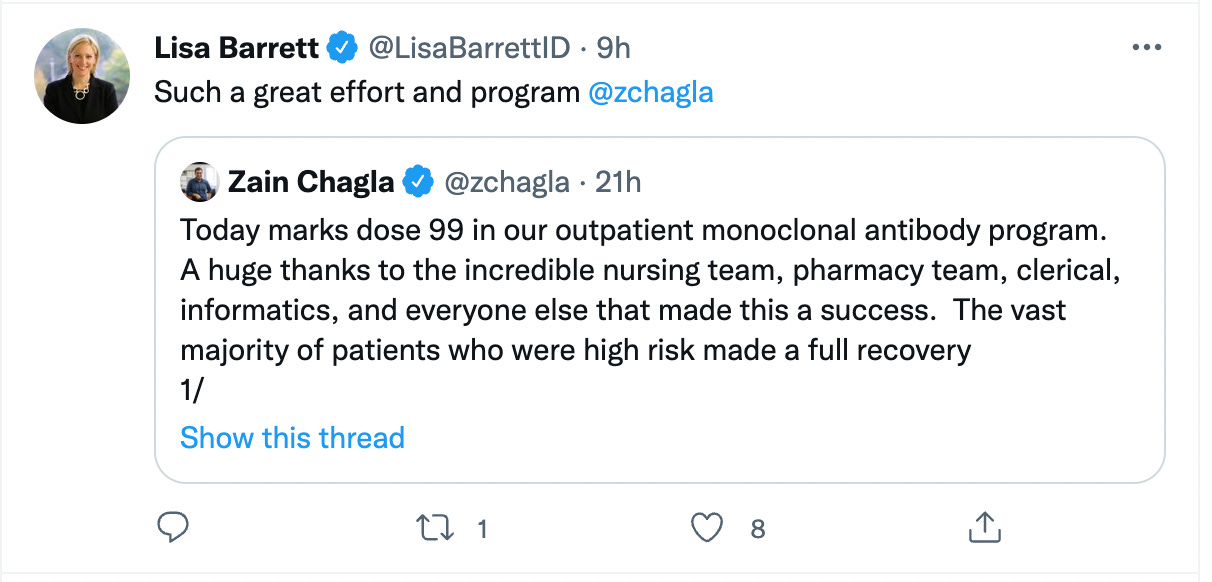
On December 27, Chagla posted about his clinic’s success on Twitter: “Today marks dose 99 in our outpatient monoclonal antibody program. A huge thanks to the incredible nursing team, pharmacy team, clerical, informatics, and everyone else that made this a success. The vast majority of patients who were at high risk made a full recovery.”
“Such a great effort and program,” tweeted Barrett.
A few days ago, I was forwarded an email that was originally sent to all the emergency room physicians in the province. The email provided a hyperlink for a screening tool, Monoclonal Antibody Eligibility and also provided a phone number for Dr. Barrett, who “has made herself available” for anyone who might want to discuss “who might benefit from this therapy.”[ii]
The email also indicated sotrovimab could “be made available through ambulatory care services” in Nova Scotia, and that “other sites for infusion” were being considered.
“While there is a timeframe for eligibility for the drug, it is generally not required that the patient receive it immediately when identified, hence giving time to make appropriate arrangements,” the email reads.
But given how OMICRON is speedily making its way around the province, I’m still stumped about why the public hasn’t been made aware of either the availability of these early treatments or their eligibility to access them. I think people should be made aware of them. There should be an online tool, as well as a news release indicating the new recommendation for the drug.
As of January 11th, 34 people in Nova Scotia have been treated with sotrovimab, with enough supply to treat 255 more.
This seems to be a big improvement. During the 4th wave, 20 doses of casirivimab/imdevimab were available and 4 people were treated. During the 3rd wave, 150 doses of bamlanivimab were available, and only one person was treated.
~
While the focus of my research over the last several months has been with how Nova Scotia has dealt with its supply of these early treatments, other provinces do not appear to have fared much better. It should be noted that even though the federal government has authorized and purchased the drugs, their use falls under the practice of medicine—a provincial and territorial jurisdiction.
Early on, a noticeably frustrated Dr. Carl Hansen, the CEO of AbCellera Biologics, the Vancouver-based biotech firm that developed bamlanivimab told CBC Power and Politics that the drug could be saving lives in Canada but was instead sitting in storage.
That any province or territory would not make full use of a potentially life-saving and money-saving treatment during a pandemic is beyond my comprehension.
According to Chagla, in addition to saving lives, the treatments could also provide huge cost savings.
“There are operating costs of a clinic, of paying nurses, a pharmacy cost. The drug has been procured by the federal government, so for now, it’s not costing hospitals anything. But eventually [the treatment] will likely have to be bought by health care facilities. But again, you know, the average infusion likely runs, with people’s time, maybe $500 to $1,000 a day to run a clinic. A single hospitalization prevented is $23,000, and if that person goes to ICU, it costs over $50,000.”[iii]
Dr. Zain Chagla, Twitter
In terms of why the treatments were not deployed in Canada during wave 3, Chagla says, “No one had an implementation plan and health care was stretched to the limit… so it was kind of put on the back burner, even though the data was starting to suggest that it did save people from ending up in hospital.”
There is probably some truth to that. And while I’m nowhere near having any definitive answers, my sense is that some truth also lies elsewhere.
When the vaccine rollout became the public health priority, governments (both federal and provincial) didn’t want early treatments with monoclonal antibodies to be seen as a viable alternative.
This sentiment comes though over and over again in articles like this recent one about monoclonals, where the drug is referred to as a “rescue treatment,” and “not a replacement for vaccination.” Or this piece, where monoclonals are compared to an “ambulance,” while the vaccines are more like a “seatbelt.” Or this release by the US Food and Drug Administration (FDA) about the authorization of new long-acting monoclonal antibodies, but with the editorial qualifier that “vaccines have proven to be the best defense available against COVID-19,” even though for some people who are immunocompromised, monoclonals ARE actually better.
Even in this recent news release by GlaxoSmithKlein, announcing the government of Canada’s purchase of 20,000 more doses of sotrovimab, Dr. Zain Chagla felt compelled to give a nod to the vaccines: “While vaccination is a critical prevention measure to help end the COVID-19 pandemic, access to early treatments for those who are at risk to progressing to severe disease is paramount for the future.”
As vaccines wane and new variants evade current vaccine-induced immunity, it’s difficult to know who might need monoclonal treatment in the future. It could be any one of us.
At one point in a CBC interview, Chagla was asked about how he squares promoting a treatment that could be “co-opted by people who might be vaccine hesitant or anti-vaccination.”
Chagla replies:
“I think we have a duty to use what we can for patients. And again, we do this in other forms in infectious disease. You know, we have lots of preventative therapies for HIV, but it doesn’t mean we stop caring about HIV patients who contract the disease. And you know that similar paradigm has to just be put into place here. We have tools that are evidence based. We have access to them regardless of the patient population. We need to treat patients first and foremost, and that’s what health care providers do.”
Will the availability of an early treatment discourage people from getting their primary series or getting their boosters? I don’t know, but even if it did, that would be a highly unethical justification for not providing Nova Scotians with a potentially life-saving treatment.
[i] Dr. Lisa Barrett is the principal investigator (and sponsor) of The COVID Victory Study (CO-VIC), launched in June 2020 by the Nova Scotia Health Authority. The study was to take place over 18 months with the stated goal of enrolling 798 hospitalized patients, to test potential therapies and monitor their impact on COVID-19 symptoms. According to a news release, the main goal of the study was to gather information about the immune response during and after treatment and infection “to make smarter choices for the next round of vaccines.” According to the CO-VIC Web site, bamlanivimab does not appear to be included in the roster of drugs being tested. Those listed are lopinavir (an HIV medication), hydroxycholoroquine (an anti-malarial medication), and baricitinib (an anti-inflammatory). But according to the May interview with Barrett, remdesivir and tocilizumab were added to the list, and were provided to hospitalized COVID patients enrolled in her study. No mention was made of bamlanivimab ever being part of the existing protocol.
[ii] According to the eligibility criteria, except for fully vaccinated individuals who are immunocompromised, only unvaccinated individuals will be eligible. But given that nearly 70% of those admitted to hospital “due to” COVID are either double or triple vaxxed, with an average age of 65 years, I wondered why they wouldn’t just provide monoclonals to anyone who at high risk of hospitalization or serious outcomes, regardless of vaccination status? According to the Advisory Group: “The criterion is 'fully vaccinated'. While it is not explicit in the document, those individuals who are less than fully vaccinated (that would include 2 doses if they are eligible for a 3rd) are eligible for this medication. In fact, people in this province with 2 doses have been given this medication.” Since what appears to be a new definition of “fully vaccinated” was not “explicit” in the document, it therefore means it was implicit. Does this mean the definition of “fully vaccinated” is about to change from 2 doses to 3? I asked and am still waiting for an answer.
[iii] All of the quotes for Dr. Zain Chagla are taken from the October 20, 2021 interview on “The Current,” with Matt Galloway.









Great piece, and I am so glad to have the chance to read it - despite how depressing it is that you have had to self-publish such an extensively researched, expertly reported and skilfully written story.
That NS eligibility questionnaire indicates that MAB is not available for severely immunosuppressed individuals who have had three shots already?
That seems like a policy that could make a life-saving treatment inaccessible to those who might need it most. Particularly since those immunosuppressed and most vulnerable to severe illness might be those most likely to have already had three shots.
I note that is not the policy at Dr. Chagla's clinic, where "immunosuppressed" are eligible regardless of how many shots they've had.
Beyond unfathomable. Political agendas and corporate profits first, public health last. Well researched and written!